
Oxafence Active Protection Has Arrived.
Indications for Use:The ProGear Surgical Mask with Oxafence is a single-use, disposable device used to cover the nose and mouth of the wearer to protect from transfer of microorganisms, body fluids and particulates. The outer layer of the face mask is coated with methylene blue (0.47 micrograms per square cm area).
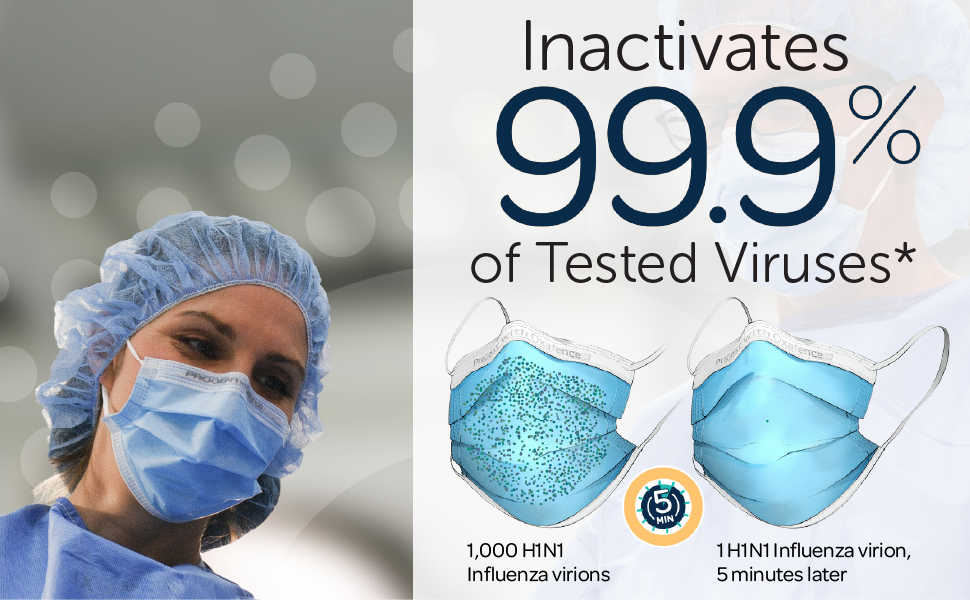
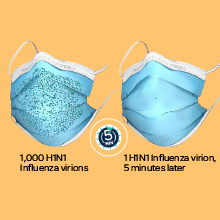
*The ProGear Surgical Mask with Oxafence inactivates single isolates of the following test viruses after five minutes of contact with the treated surface of the face mask in laboratory (in vitro) tests under a lighting intensity of 500 ± 25 lux: Influenza A/PR/8/34/H1N1 (TC adapted, ATCC VR-1469), 3 log or 99.9% inactivation; Betacoronavirus 1 OC43 (ATCC VR-1558), 4 log or 99.99% inactivation; Feline Calicivirus F-9 (ATCC VR- 782), 4 log or 99.99% inactivation. Antiviral efficacy on other viruses has not been evaluated. Correlations between in vitro testing results and any clinical event have not been evaluated.
This product is not made with natural rubber latex. Non-sterile. Do not use in MRI. If hypersensitive to Methylene Blue, consult your physician prior to use.










ProGear ASTM Level 3 Masks with Oxafence Active Protection (Box of 50 Masks)
- FDA cleared
- Made in USA
- FREE shipping on all orders

If masks can trap viruses, shouldn’t they also help inactivate them? That’s why we developed the Oxafence ProGear mask.
A lot changed in 2020 – but most masks didn't. The masks worn since then were originally designed for healthcare settings, where the focus was blocking fluids – not on addressing surface-level viral presence. We designed the Oxafence ProGear mask for today's world – one where protection matters both inside and outside clinical settings. It's built to meet the needs of everyday use, while maintaining the standards trusted by professionals. With FDA clearance and lab-tested surface-level viral inactivation*, we hope this mask helps you breathe a little easier.
Why Limiting Virus Buildup On Your Mask Matters
Even trained professionals and everyday wearers touch their masks dozens of times throughout the day. Oxafence-treated masks help reduce the risk of virus transfer during donning, doffing, and routine use. Pathogens can remain viable on untreated masks for up to 7 days. Oxafence helps inactivate tested viruses on contact – closing the gap in surface-level protection. Confidence matters. Inactivating viruses on the mask surface supports better compliance and peace of mind for frequent mask-wearers.
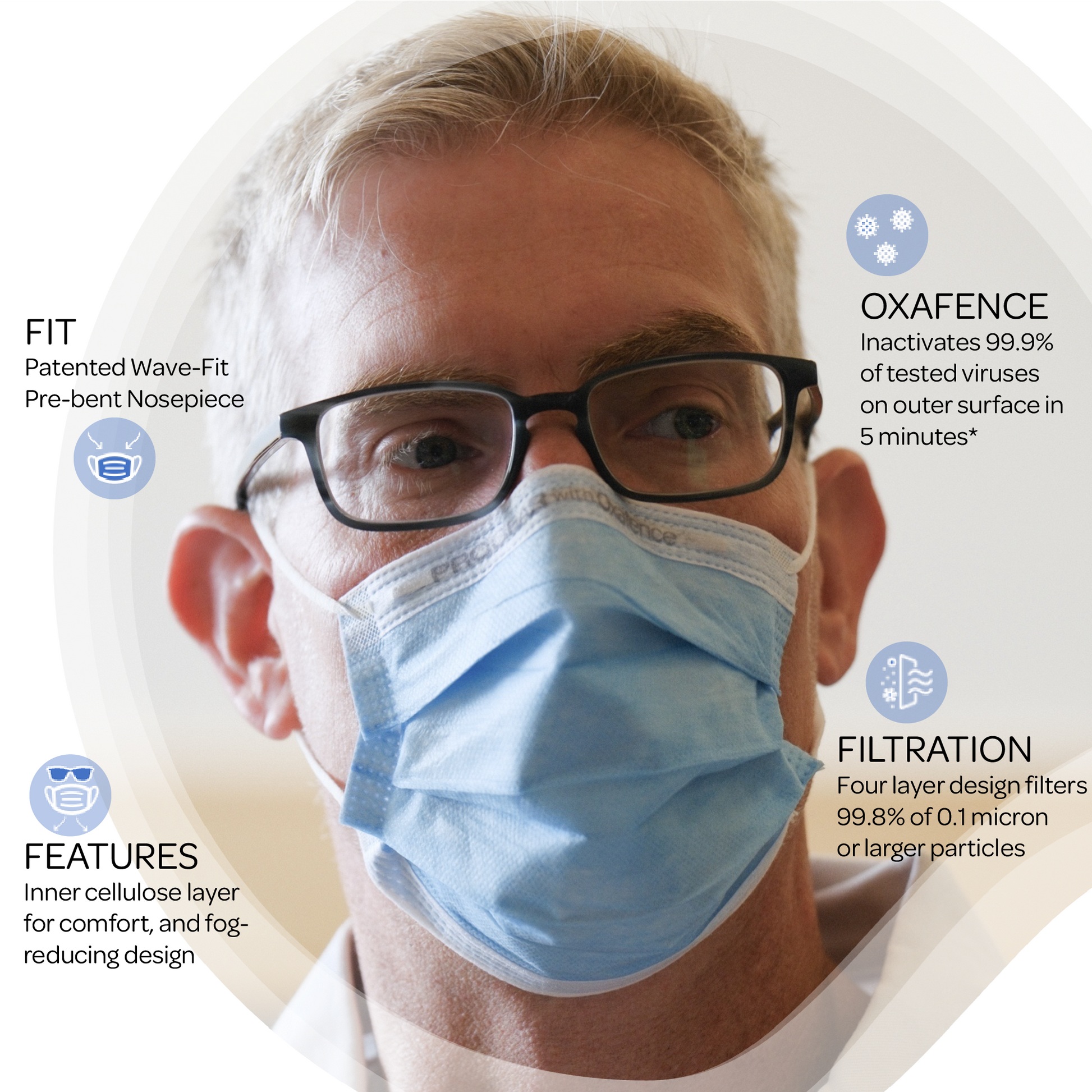
Engineered With The Features That Matter The Most

Trusted By Professionals. Designed For Everyday Use. Protection That Performs When It Matters.

Nurses & Doctors
For those who protect others.

Cancer Patients & Families
Extra reassurance when it matters most.

Retail Workers
Wear your own protection.

Travelers & Caregivers
Planes, trains, and safe visits.
Oxafence Technology Background: Science-Driven Innovation for Surface Protection
The patented Oxafence active protection layer in our ProGear surgical masks is grounded in a series of peer-reviewed studies investigating the use of light-activated methylene blue to inactivate viruses on mask surfaces.
This innovation began with a landmark study published in Infection Control & Hospital Epidemiology (2021, Lendvay et al.), co-led by Singletto co-founders Dr. Thomas Lendvay and Dr. James Chen. Conducted in collaboration with members of the WHO Coronavirus Task Force and CDC researchers, the study demonstrated that methylene blue combined with light effectively inactivated SARS-CoV-2 on medical masks while preserving their fit and function.
Subsequent research, including studies published in the American Journal of Infection Control (2022, Scholte et al.) by our scientific advisor Dr. Christopher Mores and CDC collaborators, expanded this approach to target additional high-consequence pathogens. These findings further informed the potential applications of light-activated surface protection in personal protective equipment (PPE).
In 2022, Singletto partnered with Prestige Ameritech to integrate its patented Oxafence technology into a surgical mask designed to optimize viral protection. This collaboration led to the FDA's clearance of the ProGear Surgical Mask with Oxafence, Model AV82030 (510(k) Number K231741), on September 5, 2024, under FDA product code OUK. Surgical masks classified under OUK, which include antimicrobial or antiviral agents, undergo a more stringent FDA review process compared to non-antimicrobial masks. This includes additional evaluations to ensure the safety and efficacy of the antimicrobial components, thereby providing enhanced confidence of the mask's protective capabilities.
Building upon our successful collaboration with Prestige Ameritech, Singletto is exploring opportunities to integrate its patented Oxafence Active Protection technology into a broader range of protective products with Prestige Ameritech and other strategic partners.
Looking to dive deeper into Oxafence technology? Singletto has made extended research and development data available beyond this listing through professional channels. We encourage scientifically-minded shoppers to explore further.
The Oxafence photosensitizer coating is protected under U.S. Patent Nos. 11,458,220 and 11,925,717. These patents cover antimicrobial and antiviral disinfection methods for personal protective equipment, including masks, utilizing light-activated photosensitizer formulations. Additional patents are pending.
The Wave-Fit pre-bent nosepiece, developed by Prestige Ameritech, is protected under U.S. Patent No. 8,061,356. This patent describes a directional flat face mask design that enhances fit and comfort.
What Sets Oxafence ProGear Masks Apart?
Oxafence Active Protection Technology
Oxafence inactivates 99.9% of tested virus particles on the mask surface within 5 minutes.* Correlations between in vitro testing results and any clinical event have not been evaluated.

FDA-Cleared Surgical Mask
510(k)-cleared (K231741) as a surgical mask with antimicrobial and antiviral agents, and independently tested in ISO 17025-accredited labs.

Exceeds ASTM Level 3 Standards
ASTM Level 3 Certified for high filtration, fluid resistance, and breathability.
Everyday Comfort
Soft inner lining for sensitive skin, Wave-Fit nosepiece for a contoured seal, and a fog-reducing design for clear, breathable comfort.
Healthcare
Healthcare Workers and Patients Deserve Better Protection
Are you a healthcare organization looking to bring greater protection for healthcare workers and patients?